Critical Review of Methods of Studying Fish Feeding Based on Analysis of
- Research
- Open Admission
- Published:
Safety and efficacy of volume-based feeding in critically ill, mechanically ventilated adults using the 'Protein & Energy Requirements Fed for Every Critically ill patient every Fourth dimension' (PERFECT) protocol: a before-and-after study
Critical Care volume 23, Article number:105 (2019) Cite this article
Abstract
Background
Underfeeding in critical illness is mutual and associated with poor outcomes. Co-ordinate to large prospective hospital studies, volume-based feeding (VBF) safely and finer improves energy and protein commitment to critically ill patients compared to traditional rate-based feeding (RBF) and might improve patient outcomes. A before-and-after report was designed to evaluate the safety, efficacy and clinical outcomes associated with VBF compared to RBF in a single intensive care unit of measurement (ICU).
Methods
The sample included consecutively admitted critically ill adults, mechanically ventilated for at least 72 h and fed enterally for a minimum of 48 h. The showtime accomplice (n = 46) was fed using RBF, the 2d (north = 46) using VBF, and observed for 7 days, or until extubation or decease. Statistical comparison of per centum feed volume, energy and protein delivered, plus indices of feed intolerance, were the primary outcomes of interest. Secondary observations included ventilation catamenia, mortality, and length of ICU stay (LOICUS).
Results
Groups were comparable in baseline clinical and demographic characteristics and nutrition practices. Volume delivered to the VBF grouping increased significantly by eleven.2% (p ≤ 0.001), energy by 13.iv% (p ≤ 0.001) and poly peptide by 8.4% (p = 0.02), compared to the RBF group. In the VBF group, patients coming together > 90% of free energy requirements increased significantly from 47.viii to 84.8% (p ≤ 0.001); those coming together > xc% of protein requirements changed from 56.five to 73.nine% (p = 0.134).
VBF did not increase symptoms of feed intolerance. Adapted binomial logistic regression found each boosted 1% of prescribed feed delivered decreased the odds of vomiting by 0.942 (5.8%), 95% CI [0.900–0.985], p = 0.010.
No differences in bloodshed or LOICUS were identified. Kaplan-Meier found a significantly increased extubation rate in patients receiving > 90% of poly peptide requirements compared to those meeting < 80%, (p = 0.006). Adapted Cox regression plant the daily probability of existence extubated tripled in patients receiving > 90% of their protein needs compared to the group receiving < lxxx%, adventure ratio 3.473, p = 0.021, 95% CI [1.205–10.014].
Conclusion
VBF safely and effectively increased the delivery of free energy and protein to critically sick patients. Increased protein delivery may amend extubation rate which has positive patient-centred and fiscal implications, warranting larger confirmatory trials. This investigation adds weight to the ICU literature supporting VBF, and the growing evidence which advocates for enhanced poly peptide delivery to improve patient outcomes.
Groundwork
Critically ill patients are at high risk of morbidity, bloodshed and prolonged care needs [ane]; implementing practical approaches to better outcomes is of paramount importance [2]. During critical disease, evolutionary survival mechanisms release energy from stored body tissues to fuel life-supporting tasks [iii]. The cede from torso stores is deleterious, and contributes to poor outcomes: when energy and protein is delivered to critically ill patients, this risk is ameliorated and recovery potential improved [1, 4, five].
Despite the potential benefits of nutrition therapy, feeding is stopped intermittently in 85% of critically ill patients due to essential procedures and symptoms of feed intolerance [1]. These feed stops cause patients to meet merely 40–60% of their energy and protein requirements, rather than assuasive optimal delivery and coming together the minimum 80% recommended by clinical practice guidelines (CPGs) [1, 6].
Almost intensive care units (ICUs) worldwide use an hourly 'rate-based' feeding (RBF) approach, without strategies to rectify feed deficits. Testify suggests changing from a rate- to volume-based feeding (VBF) approach helps mitigate these accrued deficits without increasing feed intolerance in medical, and some surgical ICU patients [7,viii,nine,10,11,12], and past corollary, may improve clinical outcomes. Using the VBF arroyo, instead of prescribing an hourly feeding charge per unit of, for example, 50 ml/h, a patient is prescribed 1200 ml/24-h period; systems are put in place to ensure the entire amount is delivered within 24 h.
CPGs encourage adoption of VBF in local ICU practise [1, half dozen]. The Heyland group [x,xi,12], who named their VBF protocol: The Enhanced P rotein- E nergy P rovision via the Enteral Ro u te in Critically Ill P atients (PEPuP) protocol, openly share and encourage use of their resource to facilitate change in local ICUs, and endorse adapting their protocol to local context to facilitate implementation [13].
Non all studies evidence improved outcomes when energy and poly peptide goals are accomplished. The VBF studies by Haskins et al. [eight], Taylor et al. [nine] and Heyland et al. [11] reported no pregnant difference in LOS, mortality or ventilation was enabled by VBF. Some other studies evaluating overall nutritional commitment in the critically ill population [14,15,16,17] suggest reductions in bloodshed, ventilation menstruum and LOS may be achieved when patients meet their energy and/or poly peptide goals. Others warn that coming together ICU feeding targets triggers complex biochemical processes which accept the contrary effect: increasing mortality, ventilation duration and LOS [18, 19], and recommend a less aggressive approach to feeding.
3 recent meta-analyses [xx,21,22] found no difference in bloodshed, ventilation or hospital- or ICU-LOS when patients did or did not come across their energy or poly peptide needs. The randomised controlled trials included in analyses used diverse strategies to improve nutritional delivery, such as faster feed starts and rate increase to ameliorate deficits [19], or managing gastric residual volumes or small bowel feeding. All meta-analyses found patients met sub-optimal levels of protein, which may be important. Emerging bear witness suggests that with an increased turnover of up to 80% in critical disease, ICU patients have a much larger protein need than previously accepted [23,24,25,26,27]. It is common for protein to be targeted secondary to total free energy in this patient grouping and is considered a neglected area of research [28, 29].
Deciding the optimal dose and timing of meeting the energy and protein needs of ICU patients remains controversial and bailiwick to the impact of variable influences [30]. ASPEN [ane] propose assessing for malnutrition risk, using either the Nutritional Risk Screening (NRS) 2002 or the Nutrition Risk in the Critically ill (NUTRIC) scores. NUTRIC was developed by Heyland et al. [31] to identify which ICU patients benefit nigh from nutritional back up. The score has been externally validated in large prospective observational trials and found to identify the sickest patients more than likely to accept increased morbidity and mortality [32, 33]. Testify suggests the NUTRIC score predicts free energy and protein deficits in critically ill patients, merely NRS does not [34], and that energy- and protein-related improvements in mortality are greatest in those patients with longer stay, and at highest risk calculated using NUTRIC [33, 35].
In January 2016, a Local Health Board (LHB) medical-surgical ICU multidisciplinary team commenced a prospective before-and-after study designed to compare nutritional delivery betwixt RBF and VBF. The report was designed with the principal aim of confirming the hypotheses: that changing from RBF to VBF would significantly increase the percentage of prescribed feed volume, free energy and protein delivered to adult critically sick patients, without altering feed tolerance. Secondary observations of involvement included betwixt-group comparisons of patients' outcomes including mortality, length of ICU stay (LOICUS) and mechanical ventilation.
Methods
Permissions to undertake study
The study did non crave informed patient consent: the arrangement-level quality improvement initiative was designed to undertake a minimal-chance change in feed procedure which did not exceed the boundaries of standard clinical care, and could not take place practically if prior consent were required [11, 36]. The LHB 'Research and Development' section consented to the piece of work as a service evaluation project without need to pursue upstanding review. The required University Healthcare Sciences and Medical Sciences Academics Ethics Committee approval was obtained earlier data analysis.
ICU characteristics
The adult, medical-surgical ICU is within a commune general pedagogy hospital comprising 600–700 beds. Staffing is provided in a 1-to-one nurse-to-patient ratio, and patients are overseen by a Consultant Intensivist. In the years spanning 2012–2015, quarterly ICU admissions were consistent at 181–204 patients, and average length of mechanical ventilation was iii.vii–iv.0 days [37].
Recruitment
Data collection was undertaken prospectively in consecutively admitted, adult (≥ 18 years) patients who were mechanically ventilated for 72 h or more than and fed for at to the lowest degree 48 h. The Local Health Board intensive intendance unit of measurement (LHB-ICU) does non currently utilize NUTRIC, and the 72-h duration was selected a priori as a method of sample restriction to define a level of illness acuity and longer stay.
Enteral feeding was commenced within 24 h of ventilation in stable patients. Simply patients deemed clinically advisable to receive full feeding by the medical or surgical team were included. VBF was undertaken from day 2 onwards, or in one case a patient was considered suitable to come across full-volume feeds. Patients initially aught by mouth, prescribed trophic feeding, or fed cautiously due to a chance of refeeding syndrome, were included if they were able to progress to full feeding within 72 h of ventilation.
Data was collected for up to 7 days, cessation of mechanical ventilation, death or ICU discharge, whichever occurred first.
Patients were excluded if they were pregnant, and/or were receiving parenteral or oral nutrition to limit the confounding effect of alternative nutritional support [5].
Energy and poly peptide requirements
The following principles were adhered to throughout the RBF and VBF periods.
Exterior the dietitian's working hours, the ICU used a 'starter feeding regimen' devised to closely run across the American Order for Parenteral and Enteral Nutrition (ASPEN) [1] energy and protein recommendations. Information technology used a high protein, i kcal/ml feed containing 6.26 grand of protein/100 ml for most patients (Osmolite HP [38]), or an isocaloric, lower protein, renal-conserving [39] feed for patients with established chronic kidney disease (Osmolite). The dietitian changed the feed prescription if required post-obit assessment, using ASPEN [one] or other relevant guidelines [39, 40], and prescribing additional protein supplements when indicated (Additional file one: Table S1).
Feed was progressed to target rate within 6 h of starting feed, unless prescribed trophic feeding, or considered at risk of refeeding syndrome, when feed targets were met gradually [1].
The 'PERFECT' feeding protocol
The VBF protocol was adapted from PEPuP [13] and entitled: Protein & Energy Requirements Fed for Every Critically ill patient every Fourth dimension (PERFECT); unlike PEPuP, baseline semi-elemental feeds, protein supplements and prophylactic prokinetics were non used.
The PERFECT toolkit instructed nurses how to increase feed rates, (maximum 150 ml/h) to compensate for feed-stops, and render to the initial goal rate at the beginning of the ICU 24 h period. For example, a patient prescribed 1200 ml would commence feeding at 50 ml/h at 0800 h; if the patient'due south feed was off for 8 h, they had received 400 ml of feed prior, and there remained 8 h in the day on recommencing feeding, the deficit 800 ml (1200–400 = 800) would be given over eight h at 100 ml/h (800/8). The 50 ml/h rate would recommence at 0800 h. A single finish-of-day feed bolus up to 200 ml was given to supercede remaining deficits. Boluses were not administered to jejunally fed patients.
Patients' heads were elevated to thirty–45° to reduce aspiration risk, and gastric-residual book (GRV) was checked every 4–6 h. The ICU accepts and replaces GRVs up to 500 ml, with no change in feeding charge per unit in the absence of other signs of intolerance.
Ward pedagogy was delivered by nurse-champions and the dietitian throughout December 2016 at daily and weekly team meetings. 'How to' booklets were kept at each bedside. One-to-one education and feedback was provided at the bedside, and connected advertizement hoc as required. Nursing daily documentation charts included an area to document feed deficits and corrections.
Information collection
Baseline RBF data was collected prospectively for 7 months from April 2016. PERFECT was implemented January 2017, and information again collected prospectively in consecutive admissions for 6 months.
Information included age, gender, weight, height and torso mass index (BMI) in kg/chiliad2; ideal body weight (IBW) if obese, daily energy and protein requirements, the calories and grammes of protein prescribed per kilogramme, hours without feed, and the feed-book prescribed and delivered in millilitres. The hateful daily percent of prescribed feed-volume, protein and free energy delivered (including energy from propofol) was calculated for each patient, based on minimum requirement. Each patient'southward mean daily kilocalories per kilogramme and grammes of protein per kilogramme delivered were noted. A whole 24-hour interval of '0' energy and protein delivery was included as 0% achieved.
Total episodes of witnessed airsickness (gastric contents external to rima oris) and regurgitation (gastric contents within the rima oris) were noted; the expression 'vomit' hereon includes both. Mean daily episodes for patients who vomited were calculated. Patients with diarrhoea were noted. Iii or more daily liquid stools were classified equally diarrhoea using the World Health Organisation definition [41], based on nurse perception of type 6–7 stools using the Bristol Stool Nautical chart [42].
Daily patient GRV (millilitres) and the amount replaced were recorded. Prior to commencing VBF, the ICU changed from using 8-French (Fr) and x-Fr NGTs (used in the RBF catamenia) to using 12-Fr tubes, which withdraw substantially more than GRV [43, 44], making the planned between-group comparison of aspirated volumes meaningless. Prokinetic prescription and the mean pct GRV withdrawn and replaced per patient was compared between groups.
Subgroups of patients meeting < 80%, eighty–89.9% and ≥ 90% of prescribed energy or protein were prepared for comparison, to explore whatsoever differences in clinical outcomes when patients accomplished 'over' 80% of the ASPEN guideline recommendations, or specifically exceeded this.
Mean daily morning blood glucose and insulin requirement (mmol/Fifty) per patient was noted, plus diabetes in by history equally relevant to the frequency of hyperglycaemia [9].
Clinical measures recorded from the Instance Mix Programme Database (coordinated by the Intensive Intendance National Inspect & Research Center) [45] included the Astute Physiology and Chronic Health Evaluation Two (APACHE-Two) severity of illness score, advanced mechanical ventilation (days), ICU and 60-day infirmary bloodshed (days) and length of ICU stay (LOICUS) (days: calculated from day 1 of ICU admission to when 'ready for ICU discharge' to account for delays in discharge); ICU access diagnoses were summarised by surgery, respiratory, cardiovascular and 'other' (pancreatitis, gastrointestinal, neurological, sepsis, trauma, metabolic and haematological).
Statistical analysis
Power analysis
The primary outcomes of interest were energy and poly peptide delivery and feed tolerance, while secondary observations of involvement included ICU and threescore-day mortality, ventilation menses and LOICUS.
For the primary outcomes of interest, the improvements seen in protein and energy delivered to patients in the published VBF studies were classified as a medium-to-large effect size (0.seventy) for free energy, and small-to-medium issue size (0.iv) for protein. The G*Power 3 Ability Analysis Plan [46], version three.1.ix.2, was used to bear a priori analysis for one-tailed t tests and Mann-Whitney U using the estimated consequence sizes, an α-mistake level of 0.05 and an eighty% ability. A minimum sample requirement of 37 patients per group was noted.
Information management
IBM SPSS version 22 (IBM Corp., The states, 2013) [47] was used in descriptive and inferential statistical tests unless otherwise stated. Statistical significance was accustomed at the α-mistake level ≤ 0.05; post hoc significance levels are cited in reporting.
Chiselled variables are reported as counts and percentages. These were analysed for differences in proportional frequency between RBF and VBF groups using χ 2 (chi-square) Test of Homogeneity, Test of 2 Proportions, or Fisher's verbal test when jail cell counts were less than 5. Continuous variables are described by their means and standard deviations (±) when usually distributed, or by medians and interquartile range (IQR) when non-normally distributed. Mean and median group differences were compared using independent ii-sample t tests for normally distributed data, or Mann-Whitney U for non-normal distributions.
Effect sizes are reported for percentage differences in the volume, protein and/or energy delivered between the RBF and VBF groups.
Equivalence between the VBF and RBF groups for mean episodes of airsickness was explored [48, 49] using 'ii one-sided tests' (TOST). NCSS version 11 (NCSS, LLC: USA, 2016) statistical analysis software was used with a pre-stated margin of equivalence of xx% of baseline [48]. Combining both patient groups, binomial logistic regression was used to predict the probability of airsickness, adjusted for daily mean GRV, percentage feed volume delivered, and group.
Secondary outcomes of interest: 60-twenty-four hours survival, discharge and extubation charge per unit were subjected to Kaplan-Meier and Cox Regression.
Kaplan-Meier for 60-day hospital survival used ICU admission appointment as start; censoring was based on hospital discharge alive or up to threescore days in hospital alive. For extubation charge per unit analysis, patients who died on the ICU were excluded; day 1 of intubation was the starting point; extubation up to and including 24-hour interval ten was the upshot, with ventilated patients thereafter censored. For LOICUS, censoring was undertaken after day 14.
Cox regression was adjusted for APACHE-II, group, and the per centum of energy or protein delivered; the covariate diagnosis of 'respiratory disease' was added to extubation-rate analysis [50], and BMI 25–35 kg/mii/< 25 and > 35 kg/m2 to extubation-rate and bloodshed analyses.
Other
To identify predictors of increased hateful morning blood glucose, insulin and propofol, multiple regression was adjusted for the percentage of prescribed free energy delivered, APACHE-Ii, group, BMI and/or having diabetes.
Results
Patient characteristics
Both the RBF and VBF groups comprised 46 patients. There were no significant differences between the groups' patient demographic, anthropometric and baseline clinical characteristics; other than patients in the VBF group were prescribed more propofol (288.9ml) compared to the RBF group (221.6ml) (p = 0.025), and there were more than patients with a BMI 25–35 kg/mtwo in the VBF group (65.two%) than in the RBF group (43.five%) (p = 0.036) (Tabular array one).
Nutrition practices
All patients were fed by nasogastric tube, bar one in each grouping fed by nasojejunal tube. At that place was no difference in the number of evaluable feeding days (Fig. 1), p = 0.639, or duration without feed (p = 0.379) between groups (Table 1).
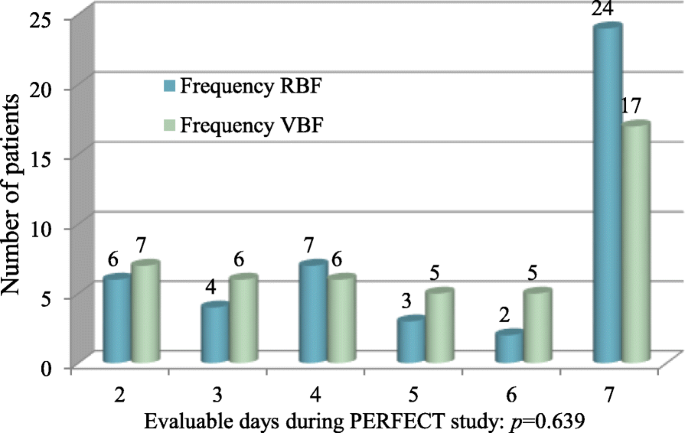
Number of evaluable feeding days in RBF and VBF groups. Effigy shows the number of evaluable feeding days, and the p value of Fisher's verbal exam demonstrating no significant group differences
There was no significant departure in the volume of feed, total energy or protein prescribed to patients in the RBF and VBF groups (Table one). Mean daily feed volume prescribed was 1312 ml (± 273 ml) in the RBF group, and 1323 ml (± 285 ml) in the VBF group, p = 0.843. Patients in the RBF group were prescribed a hateful 1755 kcal/day (± 398 kcal/twenty-four hours) and the VBF group 1787 kcal/day (± 283 kcal/twenty-four hours) p = 0.662. Daily protein prescription in the RBF group was 85.0 g/day (IQR 70.0–110.0) and ninety.v thousand/mean solar day (IQR 83–120) in the VBF group, p = 0.062.
Prescribed feed volume delivered increased by xi.2% (Table 2) from a median 87.4% in the VBF group to 98.ii% in the VBF group, p ≤ 0.001; r = 0.7 indicated a big issue size. The departure in feed volume delivered was significantly increased in the VBF group compared to the RBF group every day of the evaluable feeding period (Fig. 2). Feed book delivered besides increased significantly past 16.6% in surgical patients receiving VBF (n = 8) compared to those receiving RBF (n = 12), p = 0.001.
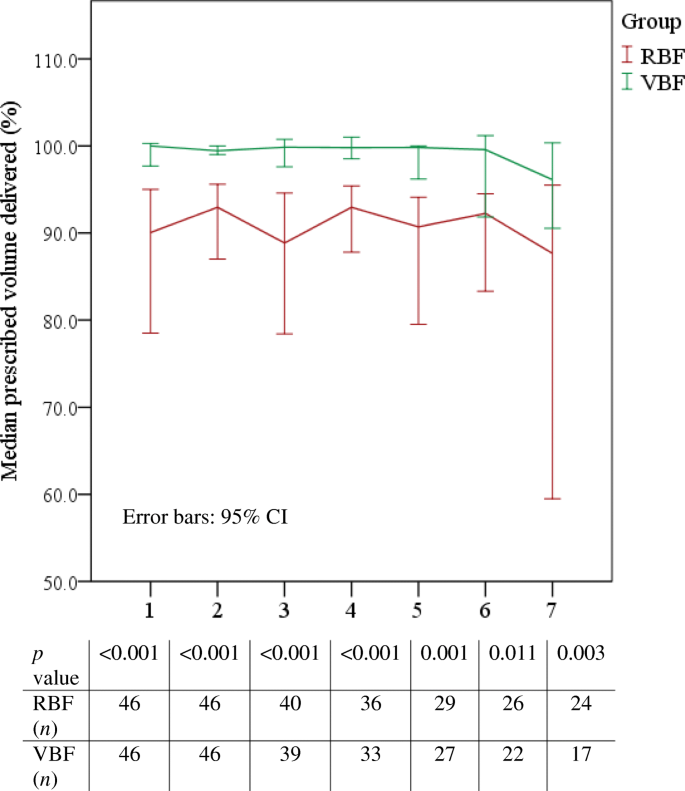
Daily median percentage feed volume delivered to RBF and VBF groups. Figure shows median 95% CI mistake bars, n per sample, and Isle of man-Whitney U upshot which establish statistically pregnant increases in book delivered to the VBF group compared to the RBF group every day
The pct of prescribed energy (Table 2) delivered increased significantly by thirteen.iv% in the VBF compared to the RBF group, from 87.9% (± 13.8%) to 101.3% (± 11.vii%), 95% CI [8.1–eighteen.7], p ≤ 0.001; eta2 = 0.22 indicated this was a large deviation. Patients in the VBF group received more than daily free energy (1785 kcal/24-hour interval, ± 245 kcal/day) than the RBF group (1541 kcal/day; ± 380 kcal/day), 95% CI [111.1–376.half-dozen], p ≤ 0.001.
The proportion of patients receiving > 90% of their energy prescription increased significantly from 47.eight% in the RBF group to 84.eight% in the VBF group (p ≤ 0.001) (Additional file 1: Table S2).
Protein delivery
Prescribed protein delivered (Table 2) increased significantly past 8.4% (p = 0.02), by a mean 20 thou per day, 95% CI [11.0–29.0], (p ≤ 0.001): from 89.2% (± 19.five%) in the RBF group to 97.half dozen% (± 14.8%) in the VBF group, 95% CI [i.ii–15.6], p = 0.02; a moderately sized difference co-ordinate to the effect size eta2 = 0.06. Subgroup analysis found patients receiving over ninety% of prescribed protein increased from 56.v% in the RBF group to 73.ix% in the VBF group, p = 0.134 (Additional file one: Tabular array S3).
Feed tolerance
There was no significant difference in patients experiencing at least 1 day of diarrhoea, with 26 in the RBF grouping (56.five%), and 18 (39.1%) in the VBF group, p = 0.095 (Tabular array iii).
The GRV replaced in the RBF (100.0%) and VBF groups (100.0%) p = 0.521 were like, and there was a non-significant difference in prokinetic prescription, with 28% of patients in the RBF group and xxx% in the VBF grouping prescribed prokinetics, p = 0.819 (Tabular array 3).
A Isle of man-Whitney U TOST identified episodes of airsickness reduced by over 20% in the VBF group (Additional file ane: Tabular array S4). The binomial logistic regression adapted odds ratio (Additional file 1: Table S5) constitute that for each boosted one% of prescribed feed delivered, in that location was a 0.942 (5.viii%), 95% CI [0.900–0.985], p = 0.010 decreased odds of airsickness.
Mean morning BG (Table 3) was significantly college in the VBF grouping (8.5 mmol/L) than the RBF group (8 .0 mmol/L), p = 0.034. Adapted multiple regression found diabetes to exist the only predictor of increased mean morning blood glucose, being ane.05 mmol/L greater in people with diabetes, than those without, 95% CI [0.456–1.646], p = 0.001 (Table 4).
There was no significant difference in the mean insulin units prescribed to patients between the RBF (median 6.seven; IQR 0.0–38.seven) and VBF (median 24.3; 0.0–39.7) groups, p = 0.248. Though not significantly different in distribution [51], the medians were notably dissimilar (Tabular array iii). Adjusted multiple regression found mean daily insulin prescription was predicted to exist xl.1 units greater in people with diabetes than those without, 95% CI, [29.013–51.163], p ≤ 0.001, and for each increment in APACHE-2 score, insulin prescription was predicted to increment by i.4 units per day, 95% CI [0.514–2.234], p = 0.003.
None of the variables, specifically grouping, BMI, percent free energy delivered or APACHE-2 score used in adjusted multiple regression, predicted propofol prescription (Table 4).
Clinical outcomes
Bloodshed
Total ICU and hospital deaths were the same or similar in each group (Boosted file 1: Table S6). Kaplan-Meier (Additional file 1: Tabular array S6; Fig. 3) and log-rank exam constitute no pregnant difference in survival distribution between groups (p = 0.693). Adjusted Cox regression (Additional file ane: Table S7) constitute neither the per centum of prescribed free energy or protein delivered nor group or BMI range predicted survival time.
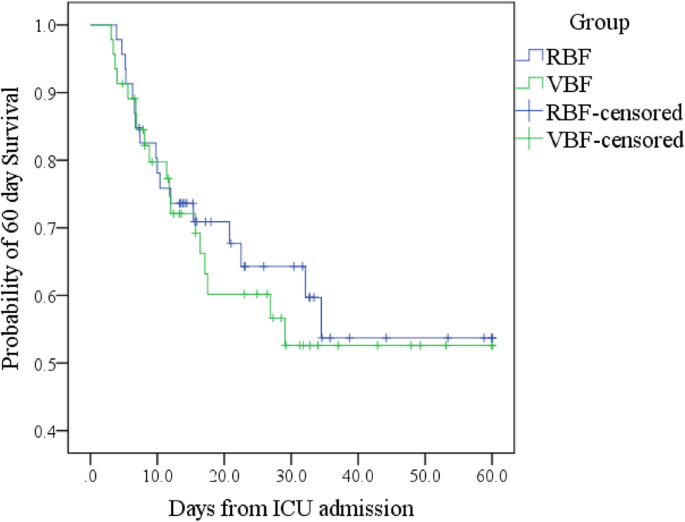
Kaplan-Meier threescore-day survival curves past grouping; shows no difference in 60-twenty-four hours survival
Mechanical ventilation
Kaplan-Meier (Fig. four; Additional file ane: Table S6) with log-rank test found patients in the RBF group had a median time to extubation of 8 days, 95% CI [4.7–eleven.3], and the VBF group had a median time of seven days, 95% CI [5.3–viii.7]; p = 0.342.
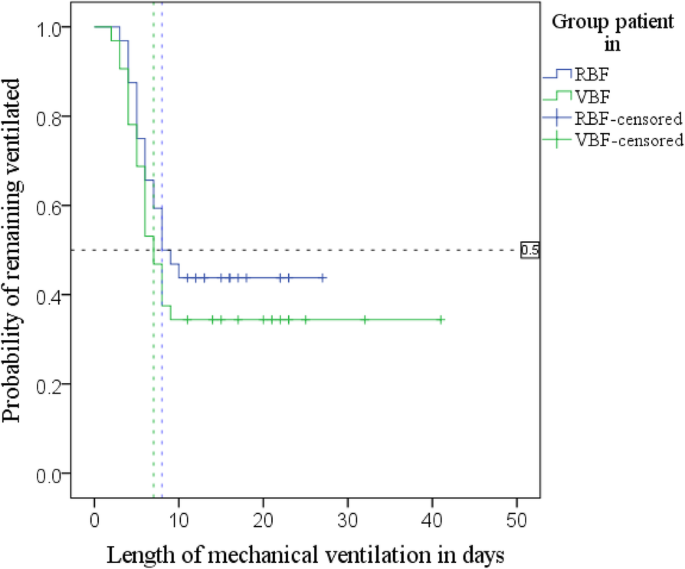
Kaplan-Meier curves; time to extubation by grouping
Adjusted Cox regression (Additional file ane: Table S7) plant that each 1% boosted protein delivery increased the daily probability of extubation 1.022-fold (by 2.two%), p = 0.040, 95% CI [ane.001–1.043]. The surviving 64 patients (minus one extreme outlier) were grouped by percent of prescribed protein delivered: < fourscore%, eighty–89.nine% and ≥ xc%. A further adjusted Cox regression (Boosted file 1: Tabular array S7) found patients receiving fourscore–89.9% of prescribed poly peptide did non have a significantly different time to extubation compared to those meeting < 80%, risk ratio (HR) one.635, p = 0.498, 95% CI [0.395–half dozen.772]; however, the daily probability of being extubated more than than tripled in patients receiving > ninety% of their protein needs compared to the grouping receiving < 80%, Hour 3.473, p = 0.021, 95% CI [1.205–10.014].
Another Kaplan-Meier was run using the same subgroups of per centum prescribed protein delivered. Kaplan-Meier curves (Fig. 5) suggested a essentially increased extubation rate in patients receiving > 90% of their protein needs; the cumulative probability of this group remaining ventilated at twenty-four hour period 10 was 24%; probability was 56% in the group meeting 80–89.ix%, and 69% in the patients who met < 80% of their poly peptide requirements.
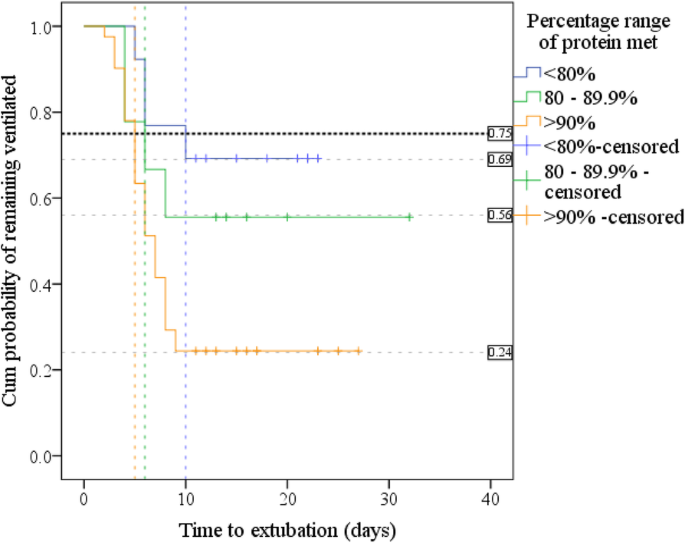
Kaplan-Meier curves for time to extubation by pct of prescribed protein delivered: shows 75th centile, days by which 25% of each group extubated and the cumulative probability (discussed as %) of remaining ventilated at twenty-four hours ten
Log-rank test found significant differences in the distribution of extubation fourth dimension between the 3 protein ranges, p = 0.012. Pairwise log-rank comparisons (Additional file 1: Tabular array S8) were undertaken to compare ventilation distributions. Using a Bonferroni correction, with significance accepted at the p < 0.0167 level, there was a significant deviation in ventilation distribution between the groups of patients receiving < 80% and ≥ xc% protein, p = 0.006.
The 75th centile is shown in Fig. 5 and Additional file i: Table S8 and demonstrates the time by which 25% of patients were extubated: 25% of those receiving < eighty% of protein were extubated past day ten, 95% CI [4.half dozen–15.4], those receiving 80–89.nine% past twenty-four hour period six, (SE not calculated), and > xc% by day 5, 95% CI [3.ix–5.9].
Length of ICU stay
Kaplan-Meier (Fig. 6; Additional file 1: Table S6) with a log-rank test establish no statistically meaning difference in the LOICUS betwixt groups, χ 2 (1) = 0.815, p = 0.367. Adjusted Cox regression identified no significant predictors of discharge rate (Additional file 1: Table S7).
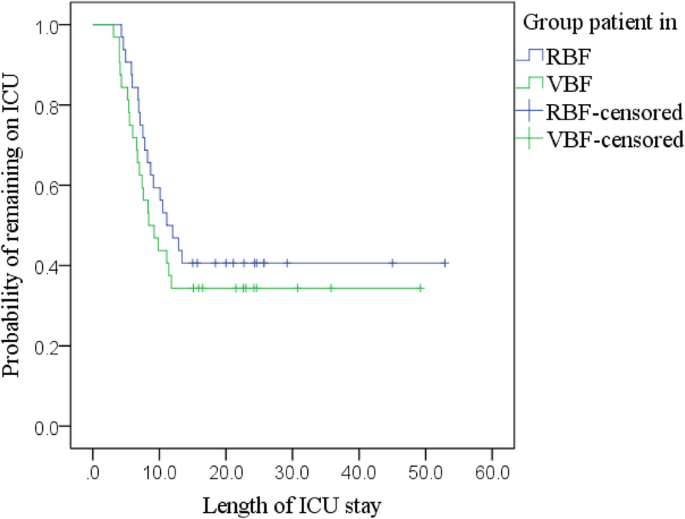
Kaplan-Meier curves: LOICUS past RBF and VBF group
Discussion
This prospective earlier-and-later on study suggests volume-based feeding safely improved patients' feed volume, energy and protein commitment, and that increasing protein delivery increased the rate of extubation, just these factors did not influence mortality or LOICUS.
Changes to delivery of feed volume, energy and poly peptide
The percent free energy increase in the VBF group compares similarly to the VBF studies in other medical-surgical ICUs which improved commitment by 9.1–17% [8, 10,11,12]. The 8.vi% protein increase achieved in the PERFECT written report was exceeded in the Heyland et al. [10,xi,12] PEPuP studies, with their improvements ranging from 11.iii to 14%, which is explained by the broad prescription of 24-g protein modules daily to patients, which were not used in the PERFECT study. In the PERFECT report, the increase in energy and poly peptide delivery in the VBF group meant that patients in this group more closely met the ASPEN diet guideline recommendations targeted by the LHB-ICU, albeit protein targets were still not achieved in the accomplice of patients with a BMI > xxx kg/yardii. The achievement of ane.3 thou of protein per kilogramme bodyweight in patients with a BMI < 29.9kg/10002 meets the minimum prescription recommended by the newly published European Society of Parenteral and Enteral Diet (ESPEN) ICU nutrition guidelines [52].
Like Taylor et al. [9], the pocket-sized subgroup of surgical patients in the PERFECT study'southward VBF group received significantly more than energy and protein than the RBF group. These findings are unlike the surgical ICU study past Declercq et al. [seven], who found no departure in energy or poly peptide delivery using VBF, which was attributed to poor protocol compliance. Heyland et al. [11, 12] acknowledge that simply using a protocol may be bereft to overcome individual unit cultural and systemic barriers. The PERFECT report utilised diverse system-level improvement techniques to facilitate adoption, such every bit identified project leadership, team coproduction, local adaptation, and staff education [53, 54]. Notably, the PERFECT report's RBF group met over 85% of prescribed energy and poly peptide needs, demonstrating the existing positive attitude to diet exercise in the LHB-ICU, which likely fostered readiness to adopt the new approach.
Feed tolerance
In that location was no difference between the RBF and VBF groups in patients experiencing diarrhoea. Taylor et al. [ix] reported the number of patients with diarrhoea significantly increased in their VBF grouping, which was attributed to large (400 ml) intragastric feed boluses not used in the PERFECT report.
Analysis found that each boosted 1% of feed volume delivered was associated with a significantly decreased odds of vomiting. This is physiologically believable given normal gastric retentivity and emptying processes [43, 55] and is supported by others' work [ix, 10, 56, 57].
Neither mean forenoon blood glucose (BG) levels nor insulin prescription was predicted by increased free energy delivery or existence in either grouping, and BG levels were maintained under 10 mmol/L as per current recommendations [one]. Obesity is independently characterised past insulin-resistance, which can be exacerbated past the added metabolic complexities of critical illness [58, 59]. Although benefits remain to be confirmed [52, 59], hypocaloric, high poly peptide feeding is proposed to minimise the metabolic consequences of over-feeding in obese patients, plus preserve nitrogen balance and lean mass [52, 58,59,60]. This approach is recommended past ASPEN [1] and was used in the PERFECT study for this patient accomplice. Managing the free energy requirements of overweight critically ill patients has not yet been addressed by research [52]. We note with interest that ESPEN [52] have very recently recommended a more conservative arroyo to prescribing energy in patients with a BMI > 25 kg/gtwo. In the PERFECT report, BMI did non predict elevated blood glucose or insulin requirements, suggesting our relatively conservative arroyo to free energy prescription mitigated risk.
Clinical outcomes
Bloodshed
Unlike the PERFECT report which plant feeding improvements did non change mortality outcomes, some studies suggest improved free energy and/or poly peptide commitment improves survival [15, 17]; others advise outcomes are worse when patients run across these targets, and suggest a less aggressive approach [eighteen, xix].
An observational indicate-prevalence study of 2772 patients from 167 medical-surgical ICUs by Alberda et al. [4] found that thousand kcal/day and 30 g protein delivered were associated with reduced sixty-day mortality, though only in patients with a BMI < 25 kg/m2 and > 35 kcal/mtwo, who may be less susceptible to the earlier metabolic consequences of critical illness [ane, v]. In the PERFECT study, 43.5% of patients in the RBF group, and 65.ii% in the VBF group, had a BMI of 25–35 kg/thou2: these patients may not accept benefitted from early macronutrient manipulation, essentially limiting the likelihood of statistically finding enhanced mortality outcomes in the pocket-size number of patients with a lower or higher BMI.
It may be that patients with a lower BMI, having less reserve, benefit more than from supplementation, while heightened alterations to macronutrient utility in critically ill obese cohorts tin can make both over- and nether-feeding detrimental [1, 58, 60, 61]. This may explicate the increased mortality attributed to enhanced feeding suggested past studies such every bit Braunschweig et al. [19], who estimated and ofttimes exceeded energy requirements at 30 kcal/kg in a largely obese accomplice. Using such estimations in the astute phase of ICU admission is associated with a metabolic burden which is not recommended [ane, 58, 61, 62].
Some studies propose 'forced feeding' in the offset week of critical illness inhibits autophagy [63, 64]. The physiological process of autophagy regulates inflammation, clears toxic cell impairment and supports protein synthesis in starvation [65]. Feeding, insulin and hyperglycaemia inhibit autophagy, prompting the hypothesis that autophagy is prevented when need is greatest in disquisitional illness, leading to accelerated muscle loss.
Autophagy is influenced past the severity of oxidative stress [65], suggesting those with greater stress may be more vulnerable to autophagy inhibition caused past overfeeding. As characterised by their demand for advanced mechanical ventilation, 89.1% of patients in the PERFECT study'southward RBF group and 97.8% in the VBF group required propofol, and the VBF group received significantly more than median units. Outcomes were not worse in the PERFECT report's VBF group despite receiving more energy and propofol than the RBF group, challenging the theory of detrimental autophagy inhibition.
The role of autophagy in critical illness and feeding is poorly understood: McClave and Weijs [65, 66] argue evidence does not support underfeeding, though suggest preventing hyperglycaemia past avoiding excessive energy provision is a prudent arroyo to limit inhibition. They note 'excessive' does non equate to meeting the guideline-recommended energy and poly peptide targets utilised in the PERFECT study, suggesting patients in the more 'aggressively' fed VBF group were protected by targeting low-to-moderate energy and higher-protein feeding in all recruits, which equally summarised earlier did not cause hyperglycaemia.
Ventilation
Enhanced protein delivery significantly increased the rate of extubation of survivors, with the daily probability of being extubated more than tripling in the PERFECT study's group of patients receiving > ninety% of their protein needs compared to patients receiving < 80%. These findings were unlike Alberda et al. [4], who establish each additional 30 thousand poly peptide was not associated with more ventilator-free days (VFDs). Alberda et al. [4] only reached lx% of target protein equalling a mean 47 g/twenty-four hours compared to the PERFECT report's frequently supplemented mean 78.i–98.ane grand/day in the RBF and VBF groups; maybe the Alberda et al. [4] study's patients did not come across plenty protein to see the ventilation improvement. This might too explain the lack of improvement seen by Heyland et al. [11] in their VBF study given that patients met only 48% of energy and protein from all sources.
Studies such equally those undertaken by Alberda et al. [4] accept been criticised for undertaking separate poly peptide analyses when using fixed-ratio feeds without protein supplementation given their propensity to underfeed protein: while patients may receive 'more than', information technology could exist bereft to change outcomes [29, 67, 68].
3 meta-analyses [20,21,22] institute no divergence in bloodshed, LOS, or ventilation when patients did or did not run into their energy and protein needs; nonetheless, none of the studies included in meta-analyses accomplished more than than a mean one.1 g/kg of protein. This factor may distinguish the PERFECT study'south extubation findings from some others' piece of work. Metabolic studies suggest ane.5–ii.five g/kg protein/solar day is required for catabolic critically sick patients to reach muscle protein synthesis [28, 69]. Reduced muscle mass inevitably weakens function and has been linked to reduced respiratory power and prolonged ventilation [66, 69], so the ventilation comeback seen in the PERFECT report is plausible and compares to some others' findings [5, 60].
Elke et al. [5] completed secondary analysis using pooled data from the Alberda et al. [4] and Heyland et al. [10] studies, to achieve a sample of 2270 patients, who, like the PERFECT study, were ventilated for over 72 h and exclusively enterally fed. Patients' nutrition was carve up into tertiles of achievement and analysed in separate logistic and linear regression models for energy and protein, adjusted for age, BMI and APACHE-Two. The study found each boosted 1000 kcal/twenty-four hour period and 30 g protein reduced 60-twenty-four hours mortality and increased VFDs. In sensitivity assay of survivors fed for the first vii days of admission, ventilation improvements only persisted for increased protein provision.
With a mean historic period 62 years, BMI 27.six kg/one thousandii, median ventilation of eight.four days, mortality 31%, LOICUS 11.v days and existence predominantly male, Elke et al. [five] argue their study is reflective of typical ICU populations and is certainly comparable to the PERFECT study's recruits.
Hoffer and Bistrian [28] suggest patients with an ICULOS longer than 3.eight days volition particularly do good from additional poly peptide to alleviate musculus cloudburst. This may be especially true for patients with pre-existing atrophy, such equally malnourished and/or inactive elderly and obese patients who depend more on feeding given their reduced ability to achieve nitrogen-balance [28]. Patients in the PERFECT and Elke et al. [five] studies were included for a minimum of three-twenty-four hour period ventilation and largely fit this description.
Strengths and limitations
The strengths and limitations of the PERFECT study require consideration.
The sample of 46 patients per group exceeded the minimal sample requirement identified through power analysis, which gives conviction in the findings suggesting feed volume, energy and protein commitment was significantly increased in the VBF compared to the RBF grouping, without adverse effects.
Heyland et al. [12] questioned whether VBF improvements in feed commitment could be reproduced using polymeric, rather than semi-elemental feeds. The PERFECT study strongly suggests this is possible and, furthermore, safely achieved without condom prokinetics as utilised by Heyland et al. [10,11,12]. The successfully improved nutritional delivery and safety seen adds particular to the published research about volume-based feeding.
Recruiting only patients ventilated for 72 or more than hours and acquiescent to VBF within the ascertainment period ensured the written report represented patients with a prolonged ICU stay and had some uniformity of disease acuity [69]. The RBF and VBF groups were well-matched with regard to baseline clinical and demographic factors and diet practices.
All the same, issue findings require cautious interpretation. We acknowledge the misreckoning influence of disease severity and population heterogeneity challenges findings which suggest changes in outcomes are causally related to nutrition in ICU observational studies [70]. Clinical outcomes were considered observations of interest and were not subject area to ability analysis. Knowledge of which patients benefit from ICU energy and protein remains elusive, with some suggesting iii groups probable; those who do, or do not recover regardless, and those who do good, such every bit patients particularly susceptible to lean-tissue atrophy, and/or otherwise identified as loftier risk [28, 31, 71].
While word established this population might be reasonably represented in the PERFECT study, if the outcomes of many recruits occurred independently of energy and protein delivery, isolating nutrition's handling consequence in this modest sample would be very challenging, particularly when, although statistically significant, the absolute difference in energy and protein delivery between groups was relatively pocket-sized. We recognise this is especially true of mortality, with much larger observational and randomised controlled trials, designed and powered specifically to measure this outcome, still yielding alien results [52].
The suggestion that increased protein delivery predicted the probability of earlier extubation in the PERFECT written report is heady, and links to the recent telephone call to research past Hurt et al. [72] to explore protein-related improvements in short-term outcomes. The findings from the small sample were strengthened by utilize of a Bonferroni correction; nevertheless, this result must be interpreted with caution and, while encouraging, should simply be used in future hypothesis evolution.
Adjustments for the nigh pertinent covariates in regression analyses were made, just, to optimise statistical quality, but a express number were introduced; there may exist other, unadjusted misreckoning influences.
As patients were non followed upward once extubated, information technology cannot be assumed that energy and protein needs continued to be met, and inconstant intakes may accept influenced the reported outcomes.
Finally, patients in the PERFECT study were not randomised, which was justified past the nature of the system-level, quality improvement intervention [73].
Decision
The investigation found the PERFECT VBF feeding protocol significantly enhanced feed volume, energy and poly peptide delivery to prolonged, mechanically ventilated patients in the LHB-ICU, without increasing feed intolerance. The exciting finding that enhanced protein delivery may improve ventilation is considered plausible, albeit requires farther confirmatory study.
The approach is at present embedded in daily exercise on our ICU. As noted, patients in the PERFECT study were quite 'typical' of ICU admissions elsewhere, suggesting the findings will be useful to those working in like medical-surgical ICUs because adopting this approach. We recognise that our ICU patients met over 80% of poly peptide and energy targets prior to commencing VBF: one might consider VBF unnecessary in such a cohort. As delineated in Fig. 2, using a VBF strategy significantly improved the likelihood of consistently achieving daily feed targets in all patients; therefore, we do consider this approach worthwhile in facilitating optimal free energy and protein delivery regardless of baseline.
Large-scale research to demonstrate the prophylactic and efficacy of VBF in other ICU populations has merit. That said, this analysis and the resulting discussion highlight that a unifying characteristic of many studies published thus far is a failure to optimise protein delivery: this may be key to improving ICU outcomes and suggests just doing 'more of the same' is not plenty. Efforts at improving feeding in ICUs meeting depression volumes are valuable, simply these efforts should be aimed at increasing supplemented protein delivery, not just full energy.
The scientific discipline which increasingly hints at patient groups more susceptible to the benefits of improved nutrient delivery, such equally those achieving high NUTRIC scores, indicates a useful research target to trial VBF. Targeting such a group would help overcome the difficulty of ascribing credit to nutrition in improving outcomes in such a heterogeneous population. The evidence from NUTRIC studies and then far does non show that feeding harms patients at depression risk with short stays, and as it is oftentimes hard to predict those who will have the longer ICU course, Compher et al. [35] endorse continuing to optimise feeding in all patients, which this investigation has shown, is enabled past the PERFECT feeding protocol in our ICU. Equally noted, we do not summate the NUTRIC score at present; this will exist a recommendation for our Unit and then we can be prepared to answer to emerging findings.
To optimise interpretation and generalisability, large, multicentre randomised controlled trials must be designed to measure outcomes related to improved protein delivery, using adequately powered samples for pre-specified event sizes [74], a priori determined patient outcomes, and bailiwick to powerful statistical analysis.
Abbreviations
- ABW:
-
Bodily bodyweight
- APACHE-II:
-
Acute Physiology and Chronic Wellness Evaluation Two (score)
- BG:
-
Blood glucose
- BMI:
-
Body mass alphabetize
- CI:
-
Confidence interval
- CKD:
-
Chronic kidney disease
- CPG:
-
Clinical practice guidelines
- CVVHDF:
-
Continuous venovenous hemodiafiltration
- Energy:
-
Expressed as kilocalories or kcal
- g:
-
Grammes (when discussing grammes of protein)
- g/kg:
-
Grammes (of protein) per kilogramme
- GRV:
-
Gastric-residual volume
- HR:
-
Hazard ratio
- IBW:
-
Ideal bodyweight
- ICNARC:
-
Intensive Intendance National Audit & Research Center
- ICU:
-
Intensive care unit of measurement
- INTACT:
-
Intensive diet in astute lung injury: a clinical trial
- kcal :
-
Kilocalories
- kcal/solar day:
-
Kilocalories per solar day
- kcal/kg:
-
Kilocalories per kilogramme
- kg:
-
Kilogrammes
- LHB:
-
Local Health Board
- LHB-ICU:
-
Local Health Board intensive care unit
- LOICUS:
-
Length of intensive intendance unit of measurement stay
- LOS:
-
Length of stay
- One thousand :
-
Mean departure
- ml:
-
Millilitre
- mmol/Fifty:
-
Millimoles per litre
- due north :
-
Number of
- NGT:
-
Nasogastric tube
- NHS:
-
National Wellness Service
- p :
-
Value denoting statistical significance
- PEPuP:
-
Enhanced Poly peptide-Energy Provision via the Enteral Route in Critically Ill Patients
- PERFECT:
-
Poly peptide & Energy Requirements Fed to Every Critically ill patient every Time
- RBF group:
-
The grouping who received rate-based feeding
- RBF:
-
Charge per unit-based feeding/procedure
- SD:
-
Standard divergence; the symbol '±' is used throughout to represent mean standard divergence
- t :
-
t exam statistic
- U :
-
Mann-Whitney U test statistic
- VBF group:
-
The group who received volume-based feeding
- VBF:
-
Book-based feeding/process
- χ 2 :
-
Chi-square
References
-
McClave SA, Taylor Be, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the provision and assessment of nutrition back up therapy in the adult critically sick patient: Society of Critical Intendance Medicine and American Society for Parenteral diet. JPEN J Parenter Enteral Nutr. 2016;twoscore:159–211.
-
grand Lives Plus. How to guide: improving critical intendance. The Wellness Foundation: Inspiring Comeback. 2010. http://world wide web.1000livesplus.wales.nhs.britain/sitesplus/documents/1011/How%20to%20%283%29%20Improving%20Critical%20Care%20%28Feb%202011%29%20Web.pdf. Accessed twenty Aug 2017.
-
Pichard C, Oshima T, Berger MM. Energy deficit is clinically relevant for critically sick patients: aye. Intensive Intendance Med. 2015;41:335–8.
-
Alberda C, Gramlich L, Jones N, Jeejeebhoy K, Day AG, Dhaliwal R, Heyland DK. The human relationship between nutritional intake and clinical outcomes in critically sick patients: results of an international multicentre observational study. Intensive Care Med. 2009;35:1728–37.
-
Elke G, Wang M, Weiler N, Day AG, Heyland DK. Close to recommended caloric and protein intake by enteral nutrition is associated with better clinical outcome of critically ill septic patients: secondary analysis of a big nutrition database. Crit Care. 2014;18:R29.
-
Disquisitional Intendance Nutrition. Canadian clinical practice guidelines 2015: summary of revisions to the recommendations 2015. http://www.criticalcarenutrition.com/docs/CPGs%202015/Summary%20CPGs%202015%20vs%202013.pdf . Accessed nineteen May 2017.
-
Declercq B, Deane AM, Wang Yard, Chapman MJ, Heyland DK. Enhanced protein-energy provision via the enteral route feeding (PEPuP) protocol in critically ill surgical patients: a multicentre prospective trial. Anaesth Intensive Care. 2016;44:93–8.
-
Haskins IN, Baginsky Yard, Garnsky Due north, Sedghi K, Yi Southward, Amdur RL, et al. A volume-based enteral nutrition support regimen improves caloric delivery but may non upshot clinical outcomes in critically ill patients. JPEN J Parenter Enteral Nutr. 2016;41:607–11.
-
Taylor B, Brody R, Kingdom of denmark R, Southard R, Byham-Gray L. Improving enteral delivery through the adoption of the "Feed Early on Enteral Nutrition fairly for Maximum Effect (FEED ME)" protocol in a surgical trauma ICU: a quality comeback review. Nutr Clin Pract. 2014;29:639–48.
-
Heyland DK, Cahill N, Dhaliwal R, Wang Thou, Twenty-four hours AG, Alenzi A, et al. Enhanced protein-energy provision via the enteral route in critically sick patients: a unmarried eye feasibility trial of the PEP upwards protocol. Crit Care. 2010;14:R78.
-
Heyland DK, Murch 50, Cahill North, McCall Thousand, Muscedere J, Stelfox HT, et al. Enhanced protein-free energy provision via the enteral route feeding protocol in critically ill patients: results of a cluster randomized trial. Crit Care Med. 2013;41:2743–53.
-
Heyland DK, Dahliwal R, Lemieux G, Wang K, Day AG. Implementing the PEP uP protocol in critical intendance units in Canada: results of a multicentre, quality improvement study. JPEN J Parenter Enteral Nutr. 2015;39:698–706.
-
Critical Care Nutrition. PEPuP Tools. https://world wide web.criticalcarenutrition.com/resource/pepup/report-tools. Accessed 28 July 2016.
-
Rugeles S-J, Rueda J-D, Diaz C-E, Rosselli D. Hyperproteic hypocaloric enteral nutrition in the critically ill patient: a randomized controlled clinical trial. Indian J Crit Care Med. 2013;17:343–ix.
-
Tsai J-R, Alter W-T, Sheu C-C, Wu Y-J, Sheu Y-H, Liu P-L, et al. Inadequate free energy delivery during early disquisitional disease correlates with increased risk of mortality in patients who survive at least seven days: a retrospective report. Clin Nutr. 2011;30:209–14.
-
Villet S, Chiolero RL, Bollmann Doctor, Revelly J-P, Cayeux K-C, Delarue J, Berger MM. Negative impact of hypocaloric feeding and energy residual on clinical effect in ICU patients. Clin Nutr. 2005;24:502–nine.
-
Wei XW, Day AG, Ouelette-Kuntz H, Heyland DK. The association between nutritional adequacy and long-term outcomes in critically ill patients requiring prolonged mechanical ventilation: a multicentre accomplice written report. Crit Care Med. 2015;43:1569–79.
-
Arabi YM, Haddad SH, Tamim HM, Rishu AH, Sakkijha MH, Kahoul SH, Britts RJ. Most-target caloric intake in critically ill medical-surgical patients is associated with adverse outcomes. JPEN J Parenter Enteral Nutr. 2010;34:280–viii.
-
Braunschweig CA, Sheean PM, Peterson SJ, Perez SG, Freels S, Lateef O, et al. Intensive nutrition in astute lung injury: a clinical trial (INTACT). JPEN J Parenter Enteral Nutr. 2015;39:13–20.
-
Al-Dorzi HM, Albarrak A, Ferwana Thousand, Murad MH, Arabi YM. Lower versus higher dose of enteral caloric intake in developed critically sick patients: a systematic review and meta-analysis. Crit Care. 2016;20:358.
-
Choi EY, Park D-A, Park J. Caloric intake of enteral nutrition and clinical outcomes in acutely critically ill patients: a meta-analysis of randomized controlled trials. JPEN J Parenter Enteral Nutr. 2015;39:291–300.
-
Tian F, Wang Ten, Gao X, Wan X, Wu C, Zhang L, et al. Issue of initial calorie intake via enteral nutrition in critical illness: a meta-analysis of randomised controlled trials. Crit Care. 2015;19:one–thirteen.
-
da Cunha HFR, da Rocha EEM, Hissa M. Protein requirements, morbidity and mortality in critically ill patients: fundamentals and applications. Rev Bras Ter Intensiva. 2012;25:49–55.
-
Weijs PJM, Looijard WGPM, Beishuizen A, ARJ G, Oudermans-van Straaten HM. Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non-septic mechanically ventilated critically ill patients. Critical Intendance. 2014;18:701.
-
Allingstrup MJ, Esmailzadeh North, Wilkens Knudsen A, Espersen Yard, Hartvig Jensen T, Wiis J, Perner A, Kondrup J. Provision of protein and free energy in relation to measured requirements in intensive care patients. Clin Nutr. 2012;31:462–8.
-
Weijs PJ, Stapel SN, de Groot SD, Driessen RH, de Jong Eastward, Girbes AR, et al. Optimal protein and energy nutrition decreases mortality in mechanically ventilated, critically ill patients: a prospective observational accomplice study. JPEN J Parenter Enteral Nutr. 2012;six:threescore–8.
-
Weijs PJM, Cynober Fifty, DeLegge Yard, Kreymann Thousand, Wernerman J, Wolfe RR. Proteins and amino acids are fundamental to optimal nutrition back up in critically ill patients. Crit Care. 2014;18:591. https://doi.org/10.1186/s13054-014-0591-0.
-
Hoffer JL, Bistrian BR. Nutrition in critical care: a current conundrum. F1000Research. 2016;5. https://doi.org/10.12688/f1000research.9278.1.
-
Weijs PJ, Wischmeyer PE. Optimizing free energy and protein balance in the ICU. Curr Opin Clin Nutr Metab Intendance. 2013;16:194–201.
-
Preiser JC, van Zanten AR, Berger MM, Biolo G, Casaer MP, Doig GS, et al. Metabolic and nutritional support of critically sick patients: consensus and controversies. Crit Intendance. 2015;xix:35.
-
Heyland DK, Dhaliwal R, Jiang X, Solar day AG. Identifying critically ill patients who benefit the virtually from diet therapy: the development and initial validation of a novel risk assessment tool. Crit Care. 2011;xv:R268.
-
Kalaiselvan MS, Renuka MK, Arunkumar AS. Apply of Nutrition chance in critically sick (NUTRIC) score to appraise nutritional gamble in mechanically ventilated patients: a prospective observational study. Indian J Crit Care Med. 2017;21:253–half-dozen.
-
Rahman A, Hasan RM, Agarwala R, Martin C, Day AG, Heyland DK. Identifying critically-ill patients who will benefit most from nutritional therapy: further validation of the "modified NUTRIC" nutritional risk assessment tool. Clin Nutr. 2016;25:158–62.
-
Canales C, Elsayes A, Yeh DD, Belcher D, Nakayama A, McCarthy CM, et al. Nutrition Risk in Critically Ill Versus the Nutritional Take chances Screening 2002: are they comparable for assessing gamble of malnutrition in critically sick patients? JPEN J Parenter Enteral Nutr. 2019;43:81–vii.
-
Compher C, Chittams J, Sammarco T, Nicolo M, Heyland DK. Greater protein and free energy intake may be associated with improved mortality in higher risk critically ill patients: a multicentre, multinational observational study. Crit Care Med. 2017;45:156–63.
-
Fiscella K, Tobin JN, Carroll JK, He H, Ogedegbe G. Upstanding oversight in quality improvement and quality comeback inquiry: new approaches to promote a learning wellness care system. BMC Med Ethics. 2015;sixteen:63.
-
WardWatcher™ Software (2013–2015). Critical Care Audit Ltd, Yorkshire, United kingdom. http://www.sicsag.scot.nhs.uk/Data/WardWatcher.html. Accessed 12 Aug 2016.
-
Abbott Nutrition. Tube feeds. https://www.abbottnutrition.co.uk/products-and-services/abbott-nutrition-products/tube-feeds/. Accessed 11 November 2017.
-
Kidney Affliction: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical do guideline for the evaluation and direction of chronic kidney illness. Kidney inter. 2013;three:i–150.
-
Henry CJ. Basal metabolic charge per unit studies in humans: measurement and development of new equations. Public Health Nutr. 2005;8:1133–52.
-
World Health System. The handling of diarrhoea: a manual of physicians and other senior health workers. Geneva: World Health Organisation; 1995.
-
Heaton KW, Lewis SJ. Stool form scale every bit a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–4.
-
Guo B. Gastric balance volume management in critically sick mechanically ventilated patients: a literature review. Proc Singapore Healthc. 2015;24:171–lxxx.
-
Metheny NA, Schallom 50, Oliver DA, Clouse RE. Gastric residual volume and aspiration in critically ill patients receiving gastric feedings. Am J Crit Intendance. 2008;17:512–nine.
-
Intensive Care National Audit & Research Heart (ICNARC). https://www.icnarc.org/. Accessed 15 Dec 2018.
-
Faul F, Erdfelder E, Lang A-G, Buchner A. G*Ability 3: a flexible statistical power analysis plan for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91.
-
IBM Corp. IBM SPSS statistics for windows, version 22.0. Armonk: IBM Corp; 2013.
-
Riffenburgh RH. Statistics in medicine. tertiary ed. London: Bookish Press; 2012.
-
Walker E, Nowacki Equally. Understanding equivalence and noninferiority testing. J Gen Intern Med. 2011;26:192–half-dozen.
-
Kulkarni AP, Agarwal Five. Extubation failure in intensive intendance unit: Predictors and direction. Indian J Crit Care Med. 2008;12:1–nine.
-
Hart A. Mann-Whitney test is not but a test of medians: differences in spread tin be important. BMJ. 2001;323:391–3.
-
Singer P, Blaser AR, Berger MM, Alhazzani Westward, Calder PC, Casaer MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit of measurement. Clin Nutr. 2018;38:48–79.
-
Taylor MJ, McNicholas C, Nicolay C, Darzi A, Bell D, Reed JE. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23:290–8.
-
Vos L, Duckers ML, Wagner C, van Merode GG. Applying the quality improvement collaborative methodology to procedure redesign: a multiple case written report. Implement Sci. 2010;5. https://doi.org/ten.1186/1748-5908-v-19.
-
Rice TW. Gastric residual volume: cease of an era. JAMA. 2013;309:283–4.
-
Juvé-Udina ME, Valls-Miró C, Carreño-Granero A, Martinez-Estalella G, Monterde-Prat D, Doningo-Felici CM, et al. To return or to discard? Randomised trial on gastric remainder volume management. Intensive Crit Intendance Nurs. 2009;25:258–67.
-
Mentec H, Dupont H, Bocchetti One thousand, Cani P, Ponche F, Bleichner G. Upper digestive intolerance during enteral diet in critically sick patients: frequency, adventure factors, and complications. Crit Care Med. 2001;29:1955–61.
-
McClave SA, Kushner R, Van Way CW, Cave M, DeLegge One thousand, Dibaise J, et al. Nutrition therapy of the severely obese, critically sick patient: summation of conclusions and recommendations. JPEN J Parenter Enteral Nutr. 2011;35 (suppl five):88S–96S.
-
Secombe P, Harley South, Chapman M, Aromataris E. Feeding the critically ill obese patient: a systematic review protocol. JBI Database Arrangement Rev Implement Rep. 2015;13:95–109.
-
Dickerson RN, Boschert KJ, Kudsk KA, Dark-brown RO. Hypocaloric enteral tube feeding in critically ill obese patients. Diet. 2002;eighteen:241–6.
-
Dickerson RN, Medling TL, Smith AC, Maish GO, Croce MA, Minard G, Brown RO. Hypocaloric, high-protein nutrition therapy in older vs younger critically ill patients with obesity. JPEN J Parenter Enteral Nutr. 2013;37:342–51.
-
Berger MM, Pichard C. Understanding the causes of death in INTACT by Braunschweig et al. JPEN J Parenter Enteral Nutr. 2015;39:144.
-
Puthucheary ZA, Rawal J, McPhail Grand, Connolly B, Ratnayake Thou, Chan P, et al. Acute skeletal muscle wasting in critical disease. JAMA. 2013;310:1591–600.
-
Schellekens W-JM, van Hees HWH, Doordiun J, Roesthuis LH, Scheffer GJ, van der Hoeven JG, Heunks LMA. Strategies to optimize respiratory muscle part in ICU patients. Crit Intendance. 2016;20:103.
-
McClave SA, Weijs PJM. Preservation of autophagy should non directly nutrition therapy. Curr Opin Clin Nutr Metab Care. 2015;18:155–61.
-
Weijs PJM. Fundamental determinants of protein requirement in the ICU. Curr Opin Clin Nutr Metab Care. 2014;17:183–ix.
-
Conduct DE, Wandrag L, Merriweather JL, Connolly B, Hart N, MPW C, on behalf of the Enhanced Recovery Subsequently Critical Illness Programme Group (ERACIP) Investigators. The office of nutritional back up in the physical and functional recovery of critically sick patients: a narrative review. Crit Care. 2017;21:226.
-
Heyland DK, Cahill N, Day AG. Optimal amount of calories for critically ill patients: depends on how you lot slice the cake! Crit Care Med. 2011;39:2619–25.
-
Nicolo M, Heyland DK, Chittams J, Sammarco TY, Compher C. Clinical outcomes related to protein delivery in a critically sick population: a multicenter, multinational observation study. JPEN J Parenter Enteral Nutr. 2015;40:45–51.
-
Sjoding MW, Luo Chiliad, Miller MA, Iwashyna TJ. When do confounding by indication and inadequate take chances adjustment bias critical care studies? A simulation study. Crit Care. 2015;19. https://doi.org/10.1186/s13054-015-0923-8.
-
Rooyackers O, Rethal MS, Liebau F, Norberg A, Wernerman J. High protein intake without concerns? Crit Intendance. 2017;21:106.
-
Hurt RT, McClave SA, Martindale RG, Ochoa Gautier JB, Coss-Bu JA, Dickerson RA, et al. Summary points and consensus recommendations from the international protein summit. Nutr Clin Pract. 2017; 32 (Suppl one):142S–151S.
-
Lee ZY, Barakatun-Nisak MY, Airini IN, Heyland DK. Enhanced protein-energy provision via the enteral route in critically ill patients (PEP uP protocol): a review of evidence. Nutr Clin Pract. 2015;31:68–79.
-
Arabi YM, Casaer MP, Chapman K, Heyland DK, Ichai C, Marik PE, et al. The intensive care medicine enquiry agenda in diet and metabolism. Intensive Intendance Med. 2017;43:1239–56.
Acknowledgements
Thank you lot to all the doctors, and especially to the nurses who contributed to and supported the work; to our clerk who assisted with information collection, and thank y'all to Graham Clarke for mentoring through statistical analysis and dissertation.
Funding
None to declare.
Availability of data and materials
The datasets used and/or analysed during the electric current study are available from the corresponding author on reasonable request.
Author information
Affiliations
Contributions
VC is a Consultant on our unit of measurement and was supportive in designing the evaluation and the final feeding process. GC is a Senior Lecturer and Teaching Fellow at Bangor University. He was my academic supervisor for my Masters Dissertation. He provided guidance and support throughout statistical analysis and marked the work in its entirety. All authors read and approved the final manuscript and agreed to be answerable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ideals approving and consent to participate
This is discussed in 'methods' as follows:
The report did not require informed patient consent: the organisation-level quality comeback initiative was designed to undertake a minimal-risk change in feed process which did not exceed the boundaries of standard clinical care, and could not take place practically if prior consent were required [11, 23]. The Local Wellness Board 'Research and Development' department consented to the piece of work as a service evaluation project without demand to pursue upstanding review. The required University Healthcare Sciences and Medical Sciences Academics Ethics Commission approving was obtained before data analysis.
Consent for publication
Not applicative.
Competing interests
The authors declare that they have no competing interests.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Boosted file 1:
Table S1. Computing patients' energy and protein prescription (using ASPEN guidelines [i] unless otherwise stated). Tabular array S2. χ2 test of homogeneity showing group frequency distributions for the percentage range of free energy delivered. Table S3. χ2 test of homogeneity showing group frequency distributions for the percentage range of protein delivered. Tabular array S4. Equivalence TOST: daily episodes of vomiting. Table S5. Binomial logistic regression predicting odds of vomiting for mean GRV, percent feed delivered, and group. Table S6. Summary of analysis for ICU and infirmary mortality, ventilation menstruation and LOICUS by group. Table S7. Results of adjusted Cox regression for 60-day survival, length of ventilation and LOICUS. Table S8. Results of Kaplan-Meier ventilation period past percentage range of prescribed poly peptide delivered: showing 75th quartile, total events and pairwise log-rank comparisons of ventilation distribution: (significance accepted at p < 0.0167). (DOCX 24 kb)
Rights and permissions
Open Admission This commodity is distributed nether the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/past/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you requite appropriate credit to the original author(south) and the source, provide a link to the Creative Commons license, and signal if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the information made available in this commodity, unless otherwise stated.
Reprints and Permissions
Well-nigh this article
Cite this article
Brierley-Hobson, S., Clarke, G. & O'Keeffe, Five. Safety and efficacy of volume-based feeding in critically ill, mechanically ventilated adults using the 'Poly peptide & Free energy Requirements Fed for Every Critically ill patient every Time' (PERFECT) protocol: a before-and-after written report. Crit Intendance 23, 105 (2019). https://doi.org/x.1186/s13054-019-2388-7
-
Received:
-
Accustomed:
-
Published:
-
DOI : https://doi.org/10.1186/s13054-019-2388-7
Keywords
- Volume-based feeding
- Critical care
- Enteral
- Protein
- Mechanical ventilation
Source: https://ccforum.biomedcentral.com/articles/10.1186/s13054-019-2388-7
0 Response to "Critical Review of Methods of Studying Fish Feeding Based on Analysis of"
Post a Comment